airway之workflow
1)airway简介
在该workflow中,所用的数据集来自RNA-seq,气道平滑肌细胞(airway smooth muscle cells )用氟美松(糖皮质激素,抗炎药)处理。例如,哮喘患者使用糖皮质激素来减少呼吸道炎症,在该实验设计中,4种细胞类型(airway smooth muscle cell lines )用1微米地塞米松处理18个小时。每一个cell lines都有对照与处理组(a treated and an untreated sample).关于实验设计的更多信息可以查看PubMed entry 24926665 ,原始数据 GEO entry GSE52778.
2)Preparing count matrices(准备制作表达矩阵)
这个矩阵必须是原始的表达量,不能是任何矫正过的表达量(RPKM等)。因为DESeq2的统计模型在处理原始表达矩阵会展示出强大实力,且会自动处理library size differences。
2.1 Aligning reads to a reference genome
for f in 'cat files'
do
STAR --genomeDir ../STAR/ENSEMBL.homo_sapiens.release-75 --readFilesIn fastq/$f\_1.fastq fastq/$f\_2.fastq --runThreadN 12 --outFileNamePrefix aligned/$f.
done #比对 for f in 'cat files'
do
samtools view -bS aligned/$f.Aligned.out.sam -o aligned/$f.bam; done #转bam文件
done
因为按照原文献跑流程,则数据量太大,耗时间,因此在airway中只用了8个样本的一些子集,来减少运行时间及内存。
library("airway")
dir <- system.file("extdata", package="airway", mustWork=TRUE) #查看数据集所在路径
list.files(dir)
csvfile <- file.path(dir,"sample_table.csv") #文件所在绝对路径
(sampleTable <- read.csv(csvfile,row.names=1)) #读取表格
filenames <- file.path(dir, paste0(sampleTable$Run, "_subset.bam"))
file.exists(filenames) #验证这8个文件在不
library("Rsamtools")
bamfiles <- BamFileList(filenames, yieldSize=2000000) #提取2000000序列,降低时间和消耗,文件1:用于构建assay信息
seqinfo(bamfiles[1]) #查看信息
sampleTable信息:
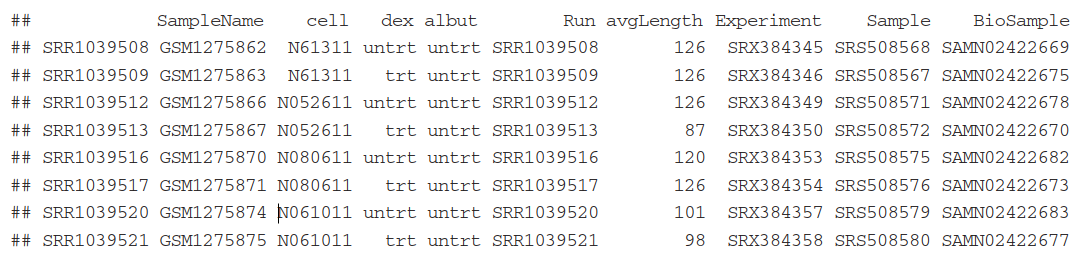
2.2 Defining gene models( 最主要的一步是构建TxDb 对象)
Next, we need to read in the gene model that will be used for counting reads/fragments.Here,we want to make a list of exons grouped by gene for counting read/fragments.
如果是自己的数据,首先下载gtf/gff3文件,并用GenomicFeatures 包中的makeTxDbFromGFF()函数构建TxDb object。(TxDb对象是一个包含frange-based objects, such as exons, transcripts, and genes)
library("GenomicFeatures")
gtffile <- file.path(dir,"Homo_sapiens.GRCh37.75_subset.gtf") #airway测试自带的gtf文件,自己的数据需要下载gtf文件
(txdb <- makeTxDbFromGFF(gtffile, format="gtf", circ_seqs=character())) #读取gtf文件构建TxDb object对象
(ebg <- exonsBy(txdb, by="gene")) #构建外显子groped by gene ,文件2:rowRanges()信息
2.3 Read counting step
上述准备工作做完之后,统计表达量非常简单。 利用GenomicAlignments包中的summarizeOverlaps()函数,返回一个包含多种信息的SummarizedExperiment 对象。同时由于数据量大,对于一个包含30 million aligned reads的单个文件,summarizeOverlaps()函数至少需要30 minutes。为了节省时间可以利用BiocParallel包中的register()函数进行多核运行。
register(SerialParam()) #设置多核运行
se <- summarizeOverlaps(features=ebg, reads=bamfiles, #创建SummarizedExperiment object with counts,上面文件1和2
mode="Union", # describes what kind of read overlaps will be counted.
singleEnd=FALSE, ######表示是pair-end
ignore.strand=TRUE, ##是否是链特异性 strand-specific
fragments=TRUE ) ##specify if unpaired reads should be counted
save(se,file='count_matrix.Rdata')
2.4 SummarizedExperiment
上述产生的SummarizedExperiment 对象,由3部分组成:粉色框是表达矩阵(matrix of counts,)储存在assay中,如果是多个矩阵可以储存在assays当中。浅蓝色框是rowRanges,包含genomic ranges的信息,及gtf中的gene model信息。绿色框包含样本信息(information about the samples)。
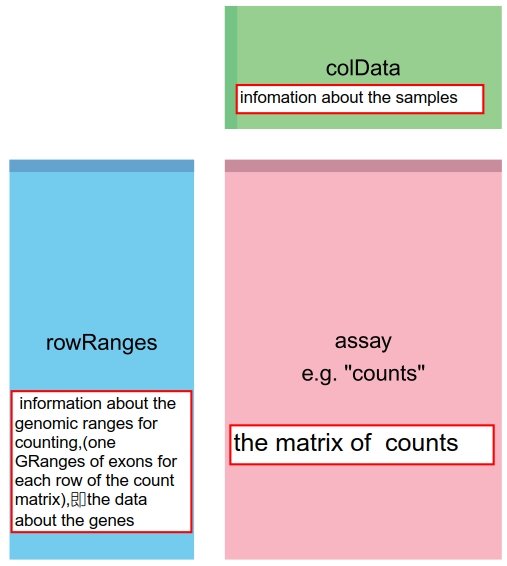
dim(se) ## [1] 20 8
assayNames(se) ## "counts"
head(assay(se), 3) #查看表达矩阵
colSums(assay(se))
rowRanges(se) #rowRanges信息
str(metadata(rowRanges(se))) #metadata是构建基因模型的gtf文件信息
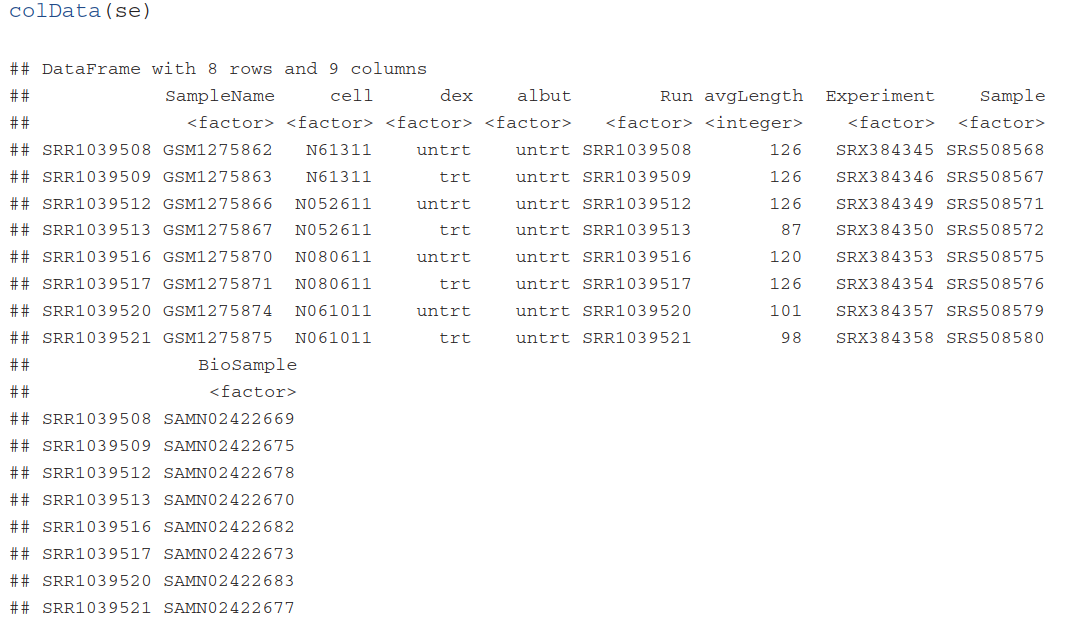
colData(se) #查看sample信息,因为还没有制作所以目前是空的
(colData(se) <- DataFrame(sampleTable)) #将样本实验设计信息传递给colData(se),文件3:colData信息。涉及到实验设计

-------------------------------------------------------------------------------------------------华丽丽的分割线----------------------------------------------------------------------
At this point, we have counted the fragments which overlap the genes in the gene model we specified。这里统计完之后,我们可以用一系列的包,进行差异分析,包括(limma,edgeR,DESeq2等)。下面我们将用DESeq2。在DESeq2中最主要的是构建 DESeqDataSet 对象,可以用上述 SummarizedExperiment objects转化为 DESeqDataSet objects。两者之间的区别是:
1)SummarizedExperiment 对象中的assay slot 被用 DESeq2中的counts accessor function代替, 且 DESeqDataSet class 强制表达矩阵中的values值为非负整数。
2)DESeqDataSet 包含design formula(设计公式)。实验设计在最初的分析中被指定,将 告诉DESeq2在分析中如何处理samples。
A second difference is that the DESeqDataSet has an associated design formula. The experimental design is specified at the beginning of the analysis, as it will inform many of the DESeq2 functions how to treat the samples in the analysis (one exception is the size factor estimation, i.e., the adjustment for differing library sizes, which does not depend on
the design formula). The design formula tells which columns in the sample information table (colData) specify the experimental design and how these factors should be used in the analysis
最简单的实验设计是 ~ condition,这里condition是 column in colData(dds),指定样本所在的分组。在airway experiment,这里我们指定实验设计为~ cell + dex表示希望检测对于不同cell line(cell). 进行dexamethasone controlling (dex,氟美松处理) 的效果。
----------------------------------------------------------------------------------------------------分割完毕-----------------------------------------------------------------------------
3、The DESeqDataSet, sample information, and the design formula
有两种方法构建 DESeqDataSet 对象:
3.1 Starting from SummarizedExperiment( 直接将SummarizedExperiment转化为DESeqDataSet )
以airway自带数据为例:
data("airway")
se <- airway
se$dex <- relevel(se$dex, "untrt") #specify that untrt is the reference level for the dex variable
se$dex
round( colSums(assay(se)) / 1e6, 1 ) #count that uniquely aligned to the genes 。round表示四舍五入,1表示小数点后一位
colData(se) #因为airway数据集制作好了这个矩阵,自己的数据需要用前面的方法制作这个。
library("DESeq2")
dds <- DESeqDataSet(se, design = ~ cell + dex) #构建DESeqDataSet对象
3.2 Starting from count matrices( 如果你之前没有准备SummarizedExperiment, 只有count matrix 和 sample information ,利用这两个文件及DESeqDataSetFromMatrix()函数构建 DESeqDataSet对象 )
countdata <- assay(se) ##这里从前面的assay中取出表达矩阵用于演示,countdata: a table with the fragment counts
head(countdata, 3)
coldata <- colData(se) ##这里从前面的assay中取出样本信息用于演示, coldata: a table with information about the samples
(ddsMat <- DESeqDataSetFromMatrix(countData = countdata,
colData = coldata,
design = ~ cell + dex) #从countdata表达矩阵和coldata样本信息构建DESeqDataSet对象
4)Exploratory analysis and visualization(数据探索分析及可视化)
4.1 Pre-filtering the dataset
nrow(dds) #64102
dds <- dds[ rowSums(counts(dds)) > 1, ]#remove rows of the DESeqDataSet that have no counts, or only a single count across all samples:
nrow(dds) #29391
4.2 The rlog transformation
需要rlog原因:
When the expected amount of variance is approximately the same across different mean values, the data is said to be homoskedastic(同方差). For RRNA-seq raw counts, however, the variance grows with the mean. For example, if one performs PCA directly on a matrix of size-factor-normalized read counts, the result typically depends only on the few most strongly expressed genes because they show the largest absolute differences between samples. A simple and often used strategy to avoid this is to take the logarithm of the normalized count values plus a small pseudocount; however, now the genes with the very lowest counts will tend to dominate the results because, due to the strong Poisson noise inherent to small count values, and the fact that the logarithm amplifies differences for the smallest values, these low count genes will show the strongest relative differences between samples.As a solution, DESeq2 offers transformations for count data that stabilize the variance across the mean. One such transformation is the regularized-logarithm transformation or rlog2. For genes with high counts, the rlog transformation will give similar result to the ordinary log2 transformation of normalized counts. For genes with lower counts, however, the values are shrunken towards the genes’ averages across all samples. Using an empirical Bayesian prior on inter-sample differences in the form of a ridge penalty, the rlog-transformed data then becomes approximately homoskedastic, and can be used directly for computing distances between samples and making PCA plots. Another transformation, the variance stabilizing transformation17, is discussed alongside the rlog in the DESeq2 vignette.
rlog()函数返回一个SummarizedExperiment对象,其中 assay slot包含 rlog-转换后的值。
rld <- rlog(dds, blind=FALSE)
head(assay(rld), 3)
par( mfrow = c( 1, 2 ) )
dds <- estimateSizeFactors(dds) #估计size factors to account for sequencing depth
plot(log2(counts(dds, normalized=TRUE)[,1:2] + 1),pch=16, cex=0.3) #then specify normalized=TRUE
plot(assay(rld)[,1:2],pch=16, cex=0.3) #Sequencing depth correction is done automatically for the rlog method (and for varianceStabilizingTransformation).

普通的log2(左)及rlog2(右)之间的比较
4.3 Sample distances
评估样本间关系(即总体相似度),哪些样本更相近,哪些更远,是否符合实验设计?
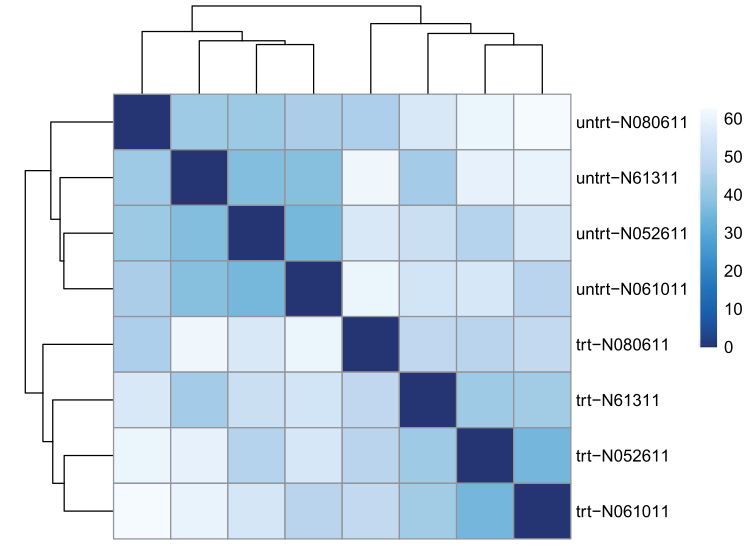
方法一:用欧式距离(Euclidean distance ),用rlog-transformed data(即normalized之后的矩阵)
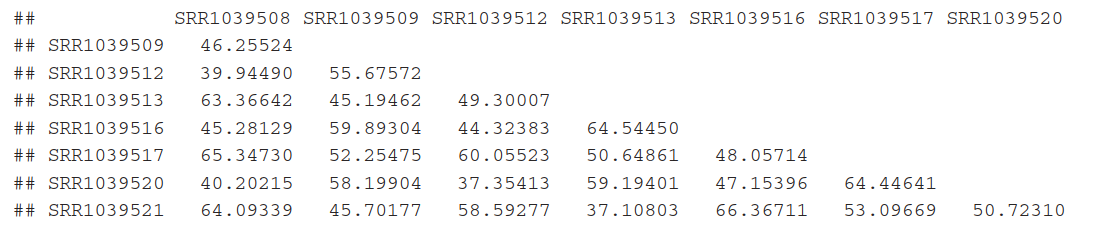
sampleDists <- dist( t( assay(rld) ) ) #欧氏距离,为确保所有基因大致相同的contribution用rlog-transformed data
sampleDists
样本间关系,欧式距离。
library("pheatmap") ##用热图展示样品间关系
library("RColorBrewer")
sampleDistMatrix <- as.matrix( sampleDists )
rownames(sampleDistMatrix) <- paste( rld$dex, rld$cell, sep="-" )
colnames(sampleDistMatrix) <- NULL
colors <- colorRampPalette( rev(brewer.pal(9, "Blues")) )(255)
pheatmap(sampleDistMatrix,
clustering_distance_rows=sampleDists,
clustering_distance_cols=sampleDists,
col=colors)
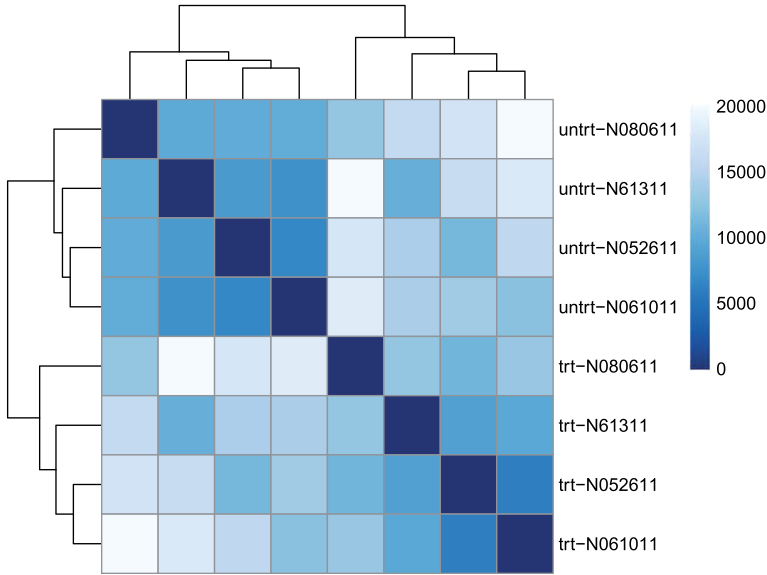
方法二:用 PoiClaClu 包计算泊松距离(Poisson Distance),必须是原始表达矩阵。The PoissonDistance function takes the original count matrix
(not normalized) with samples as rows instead of columns, so we need to transpose the counts in dds.
library("PoiClaClu")
poisd <- PoissonDistance(t(counts(dds))) ##对数据转置
samplePoisDistMatrix <- as.matrix( poisd$dd ) #计算样品间关系
rownames(samplePoisDistMatrix) <- paste( rld$dex, rld$cell, sep="-" )
colnames(samplePoisDistMatrix) <- NULL
pheatmap(samplePoisDistMatrix,
clustering_distance_rows=poisd$dd,
clustering_distance_cols=poisd$dd,
col=colors)
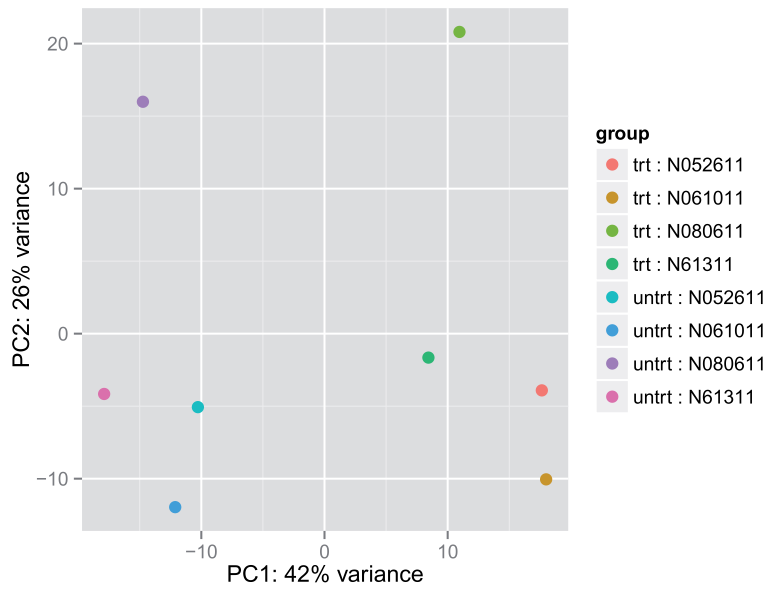
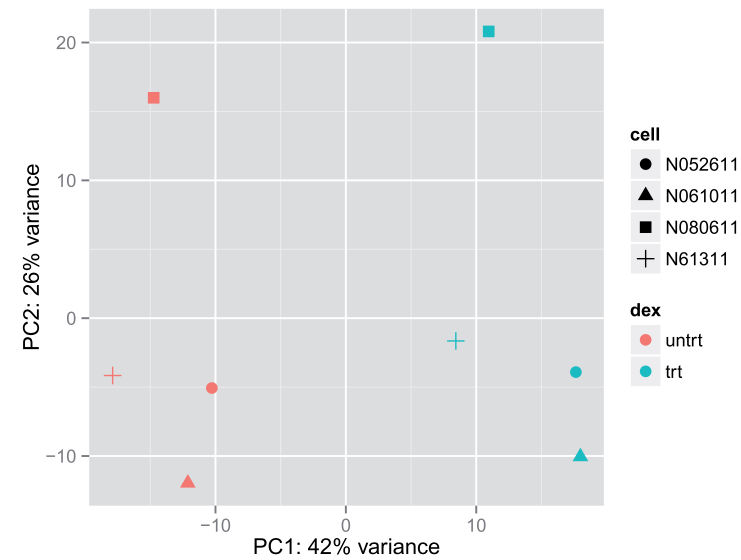
4.4 PCA plot(另一个展示样品间关系的图形)
一种方法是用DESeq2包中的plotPCA()函数
plotPCA(rld, intgroup = c("dex", "cell")) #DESeq2包plotPCA()函数
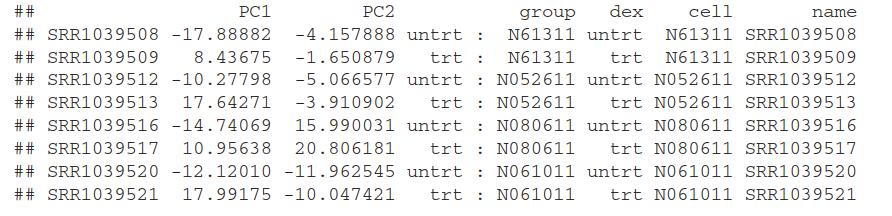
另一种方法是用ggplot2.需要调用 plotPCA()返回用于画图的数据
(data <- plotPCA(rld, intgroup = c( "dex", "cell"), returnData=TRUE)) #调用plotPCA生成要画图的data
percentVar <- round(100 * attr(data, "percentVar")) #PC1,pC2
library("ggplot2") #画图
ggplot(data, aes(PC1, PC2, color=dex, shape=cell)) + geom_point(size=3) +
xlab(paste0("PC1: ",percentVar[1],"% variance")) +
ylab(paste0("PC2: ",percentVar[2],"% variance"))
data:
4.4 MDS plot(另一种PCA图,multidimensional scaling (MDS) function )
在没有表达矩阵值,但只有一个距离矩阵的情况下使用。This is useful when we don’t have a matrix of data, but only a matrix of distances. Here we compute the MDS for the distances calculated from the rlog transformed counts and plot these。
##用归一化后的数据产生的距离矩阵(calculated from the rlog transformed)
mdsData <- data.frame(cmdscale(sampleDistMatrix))
mds <- cbind(mdsData, as.data.frame(colData(rld)))
ggplot(mds, aes(X1,X2,color=dex,shape=cell)) + geom_point(size=3) ###用原始数据产生的泊松距离矩阵(calculated from the raw)
mdsPoisData <- data.frame(cmdscale(samplePoisDistMatrix))
mdsPois <- cbind(mdsPoisData, as.data.frame(colData(dds)))
ggplot(mdsPois, aes(X1,X2,color=dex,shape=cell)) + geom_point(size=3)

5)Differential expression analysis(差异分析)
5,1)Running the differential expression pipeline
用DESeq()函数对原始表达矩阵进行差异表达分析(run the differential expression pipeline on the raw counts with a single call to the function DESeq)
dds <- DESeq(dds) #差异表达分析
5.2)Building the results table
利用result函数(),将返回一个数据框对象,包含有 metadata information(每一列的意义)。
(res <- results(dds)) #产生log2 fold change 及p值
mcols(res, use.names=TRUE) #查看meta信息
summary(res) #产看其他信息
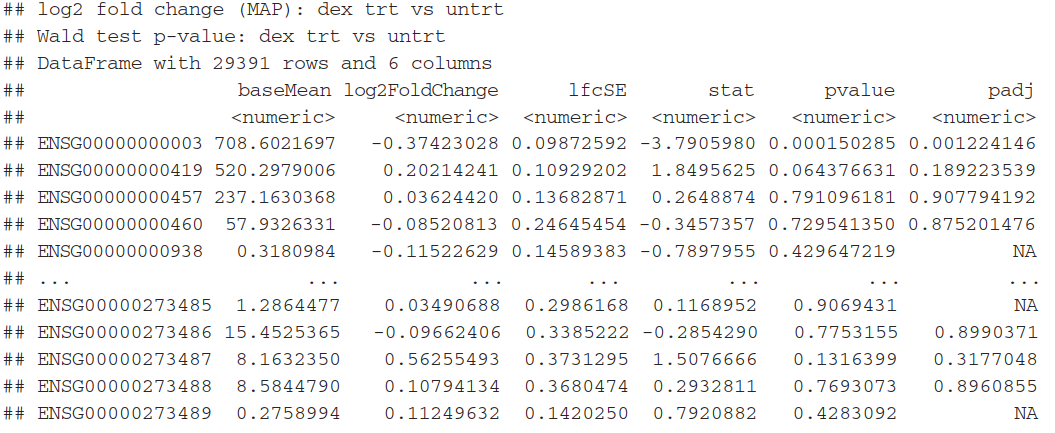
每列的含义:
baseMean:is a just the average of the normalized count values, dividing by size factors, taken over all samples in the DESeqDataSet
log2FoldChange: the effect size estimate. It tells us how much the gene’s expression seems to have changed due to treatment with dexamethasone in comparison to untreated samples
lfcSE:the standard error estimate for the log2 fold change estimate,(the effect size estimate has an uncertainty associated with it,)
p value:statistical test , the result of this test is reported as a p value. Remember that a p value indicates the probability that a fold change as strong as the observed one, or even stronger, would be seen under the situation described by the null hypothesis.
padj:adjusted p-value
2种更严格的方法去筛选显著差异基因:
降低false discovery rate threshold (the threshold on padj in the results table)
提升log2 fold change threshold(from 0 using the lfc Threshold argument of results)
#如果要降低false discovery rate threshold,将要设置的值传递给results(),使用该阈值进行独立的过滤:
res.05 <- results(dds, alpha=.05) #设置阈值FDR=0.05
table(res.05$padj < .05) #产看结果
#如果提升log2 fold change threshold show more substantial changes due to treatment, we simply supply a value on the log2 scale.
resLFC1 <- results(dds, lfcThreshold=1)
table(resLFC1$padj < 0.1)
Sometimes a subset of the p values in res will be NA (“not available”). This is DESeq’s way of reporting that all counts for this gene were zero, and hence no test was applied. In addition, p values can be assigned NA if the gene was excluded from analysis because it contained an extreme count outlier. For more information, see the outlier detection section of the DESeq2 vignette.
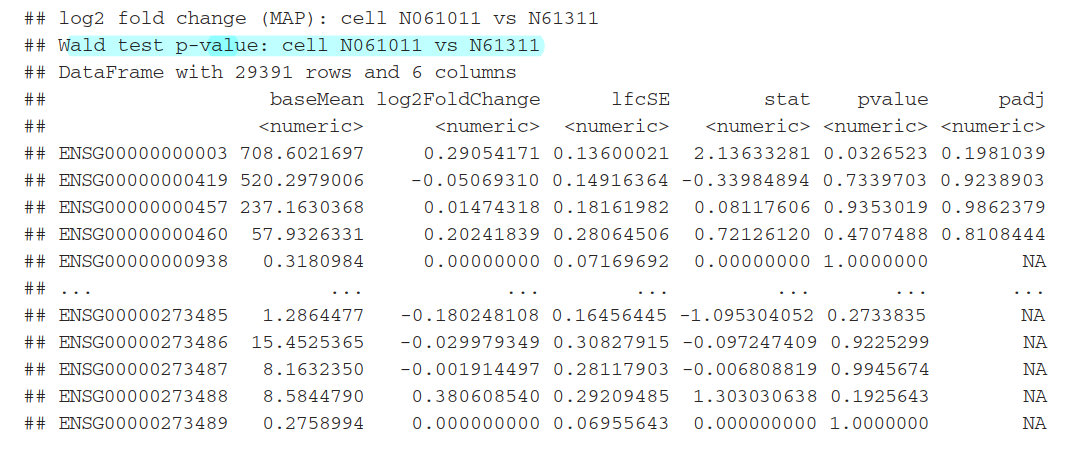
5.2)Other comparisons
可以利用result函数中的contrast参数来选择任何2组数据进行比较
results(dds, contrast=c("cell", "N061011", "N61311"))
5.3)Multiple testing(详细看文档理解FDR)
sum(res$pvalue < 0.05, na.rm=TRUE)
sum(!is.na(res$pvalue))
sum(res$padj < 0.1, na.rm=TRUE)
resSig <- subset(res, padj < 0.1)
head(resSig[ order(resSig$log2FoldChange), ])
head(resSig[ order(resSig$log2FoldChange, decreasing=TRUE), ])
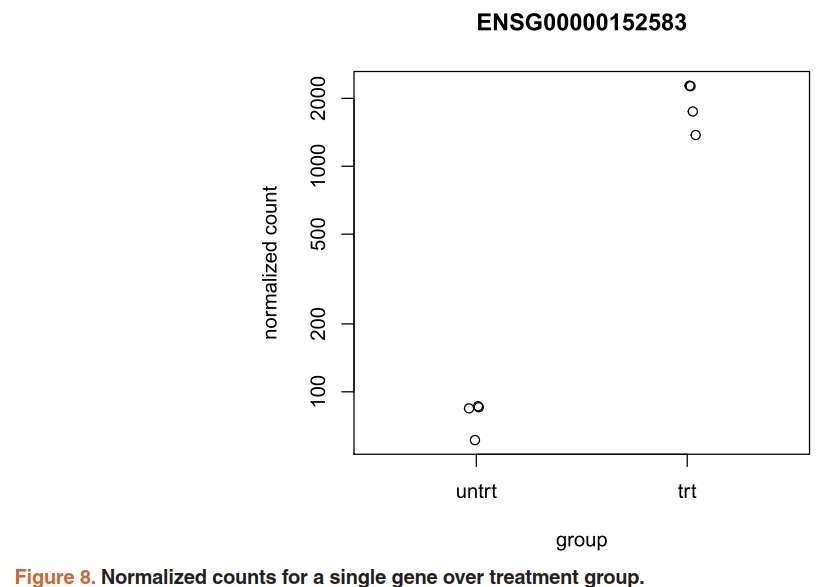
5.4)Plotting results
用 plotCounts()函数查看特定基因的表达量:需要三个参数,DESeqDataSet, gene name, and the group over which to plot the counts (Figure 8).
topGene <- rownames(res)[which.min(res$padj)]
plotCounts(dds, gene=topGene, intgroup=c("dex"))
也可以用ggplot2画传统的散点图
data <- plotCounts(dds, gene=topGene, intgroup=c("dex","cell"), returnData=TRUE)
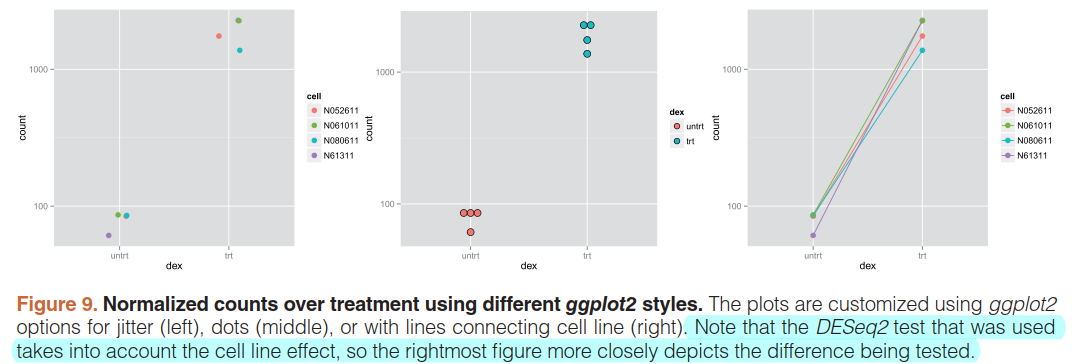
ggplot(data, aes(x=dex, y=count, color=cell)) +
scale_y_log10() +
geom_point(position=position_jitter(width=.1,height=0), size=3)
ggplot(data, aes(x=dex, y=count, fill=dex)) +
scale_y_log10() +
geom_dotplot(binaxis="y", stackdir="center")
ggplot(data, aes(x=dex, y=count, color=cell, group=cell)) +
scale_y_log10() + geom_point(size=3) + geom_line()
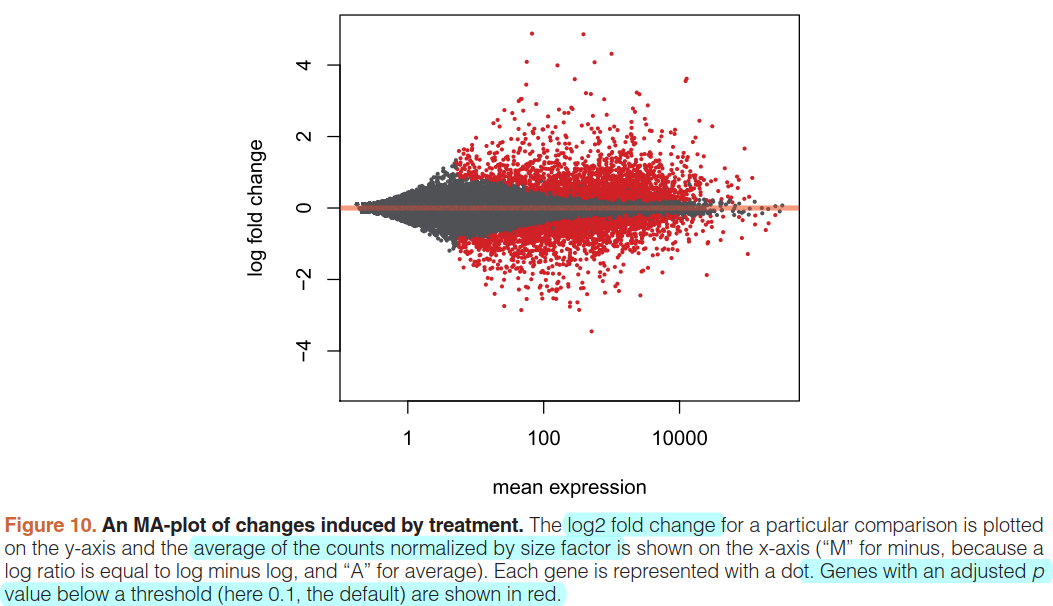
An MA-plot provides a useful overview for an experiment with a two-group comparison:
plotMA(res, ylim=c(-5,5))
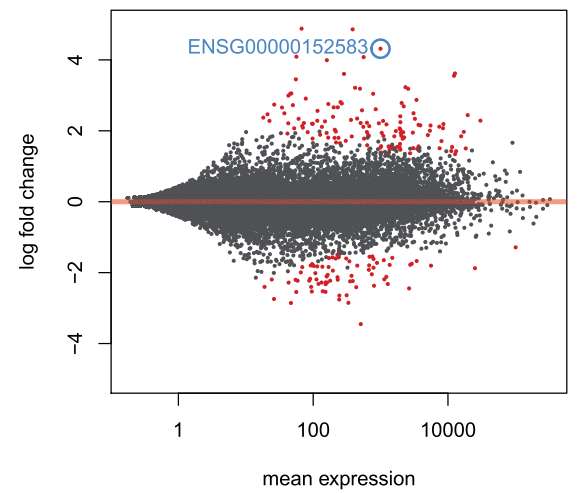
可以看到DESeq通过统计学技术,narrowing垂直方向的离散(左部分)
make an MA-plot for the results table in which we raised the log2 fold change threshold,并标记除特定的一个基因:
plotMA(resLFC1, ylim=c(-5,5))
topGene <- rownames(resLFC1)[which.min(resLFC1$padj)]
with(resLFC1[topGene, ], {
points(baseMean, log2FoldChange, col="dodgerblue", cex=2, lwd=2)
text(baseMean, log2FoldChange, topGene, pos=2, col="dodgerblue")
})
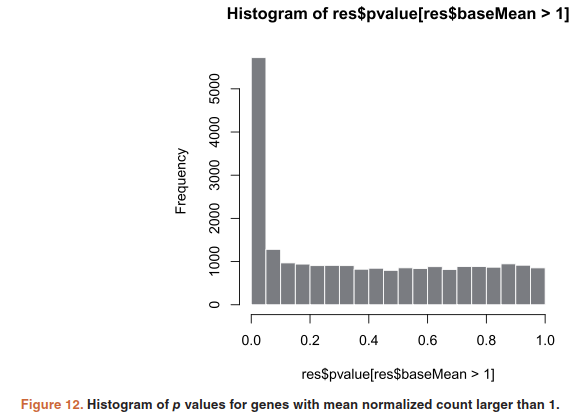
histogram of the p values:
hist(res$pvalue[res$baseMean > 1], breaks=0:20/20, col="grey50", border="white")
5.5)Plotting results
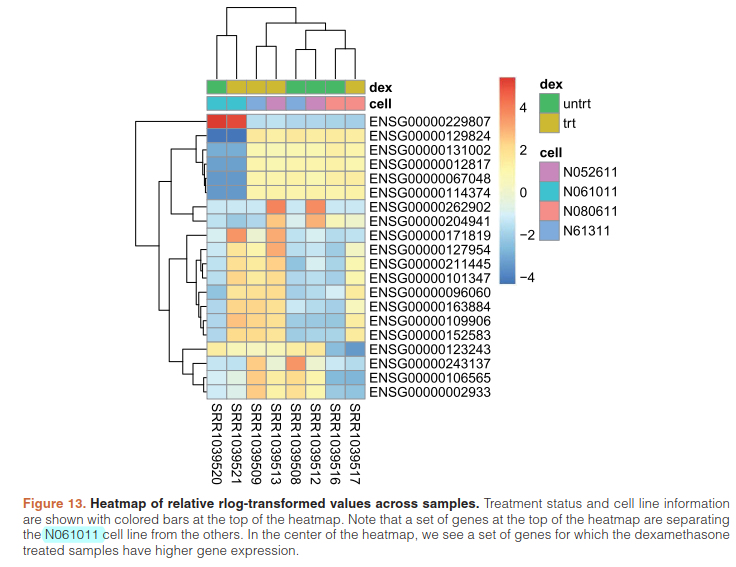
提取前20个highest variance across samples.基因进行聚类
library("genefilter")
topVarGenes <- head(order(rowVars(assay(rld)),decreasing=TRUE),20)
mat <- assay(rld)[ topVarGenes, ]
mat <- mat - rowMeans(mat)
df <- as.data.frame(colData(rld)[,c("cell","dex")])
pheatmap(mat, annotation_col=df)
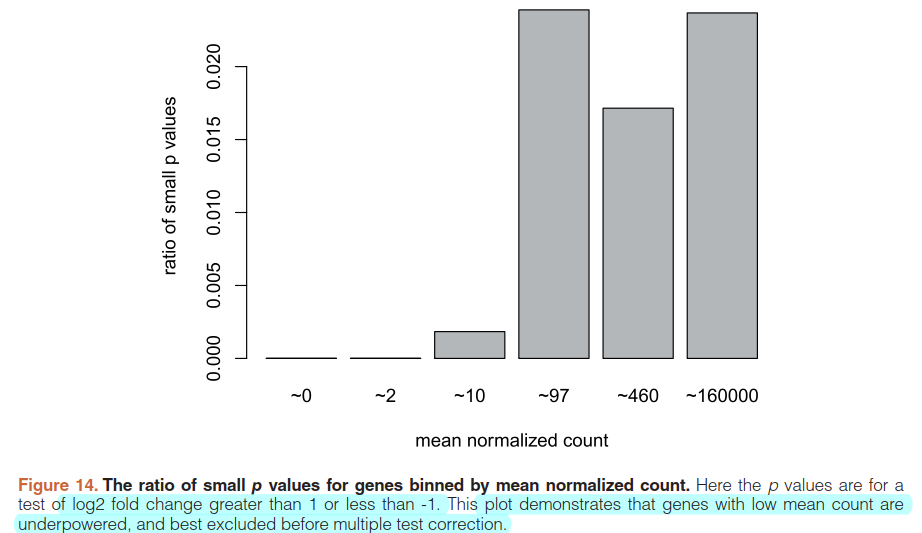
5.6)Independent filtering
qs <- c(0, quantile(resLFC1$baseMean[resLFC1$baseMean > 0], 0:6/6))
bins <- cut(resLFC1$baseMean, qs)
levels(bins) <- paste0("~",round(signif(.5*qs[-1] + .5*qs[-length(qs)],2)))
ratios <- tapply(resLFC1$pvalue, bins, function(p) mean(p < .05, na.rm=TRUE))
barplot(ratios, xlab="mean normalized count", ylab="ratio of small p values")
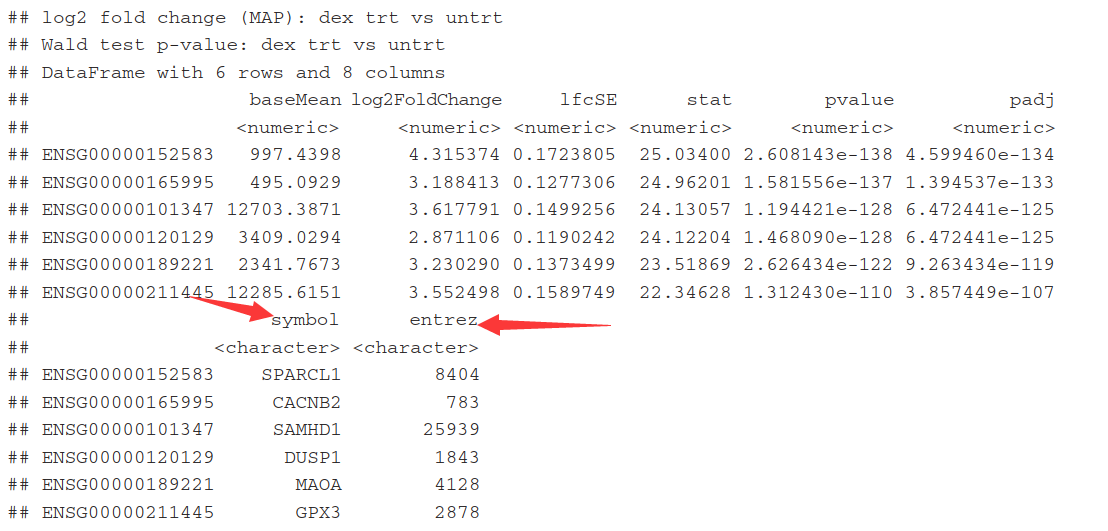
5.7)Annotating and exporting results
因为这里只有Ensembl gene IDs,而其它的名称也非常有用。用 mapIds()函数, 其中column 参数表示需要哪种信息, multiVals参数表示如果有多个对应值的时候如何选择.,这里选择第一个. 调用两次函数 add the gene symbol and Entrez ID.
library("AnnotationDbi")
library("org.Hs.eg.db")
columns(org.Hs.eg.db)
res$symbol <- mapIds(org.Hs.eg.db,
keys=row.names(res),
column="SYMBOL", #需要哪种信息
keytype="ENSEMBL",
multiVals="first") #有多个对应值,这里选第一个
res$entrez <- mapIds(org.Hs.eg.db,
keys=row.names(res),
column="ENTREZID",
keytype="ENSEMBL",
multiVals="first")
resOrdered <- res[order(res$padj),]
head(resOrdered)
5.8)Annotating and exporting results
resOrderedDF <- as.data.frame(resOrdered)[1:100,]
write.csv(resOrderedDF, file="results.csv") #保存结果 library("ReportingTools") #生成网页版结果
htmlRep <- HTMLReport(shortName="report", title="My report",
reportDirectory="./report")
publish(resOrderedDF, htmlRep)
url <- finish(htmlRep)
browseURL(url)
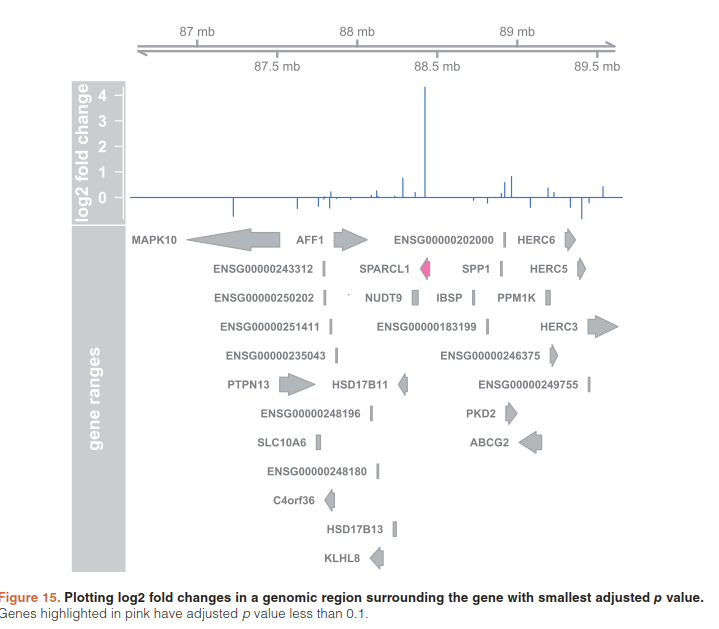
5.9)Plotting fold changes in genomic space
在基因组上展示差异结果
(resGR <- results(dds, lfcThreshold=1, format="GRanges"))
resGR$symbol <- mapIds(org.Hs.eg.db, names(resGR), "SYMBOL", "ENSEMBL") #加基因名
library("Gviz") #用于可视化for plotting the GRanges and associated metadata: the log fold changes due to dexam- ethasone treatment.
window <- resGR[topGene] + 1e6
strand(window) <- "*"
resGRsub <- resGR[resGR %over% window]
naOrDup <- is.na(resGRsub$symbol) | duplicated(resGRsub$symbol)
resGRsub$group <- ifelse(naOrDup, names(resGRsub), resGRsub$symbol) sig <- factor(ifelse(resGRsub$padj < .1 & !is.na(resGRsub$padj),"sig","notsig"))
options(ucscChromosomeNames=FALSE)
g <- GenomeAxisTrack()
a <- AnnotationTrack(resGRsub, name="gene ranges", feature=sig)
d <- DataTrack(resGRsub, data="log2FoldChange", baseline=0,
type="h", name="log2 fold change", strand="+")
plotTracks(list(g,d,a), groupAnnotation="group", notsig="grey", sig="hotpink")
5.10)Removing hidden batch effects
详细看文档
library("sva")
dat <- counts(dds, normalized=TRUE)
idx <- rowMeans(dat) > 1
dat <- dat[idx,]
mod <- model.matrix(~ dex, colData(dds))
mod0 <- model.matrix(~ 1, colData(dds))
svseq <- svaseq(dat, mod, mod0, n.sv=2)
svseq$sv
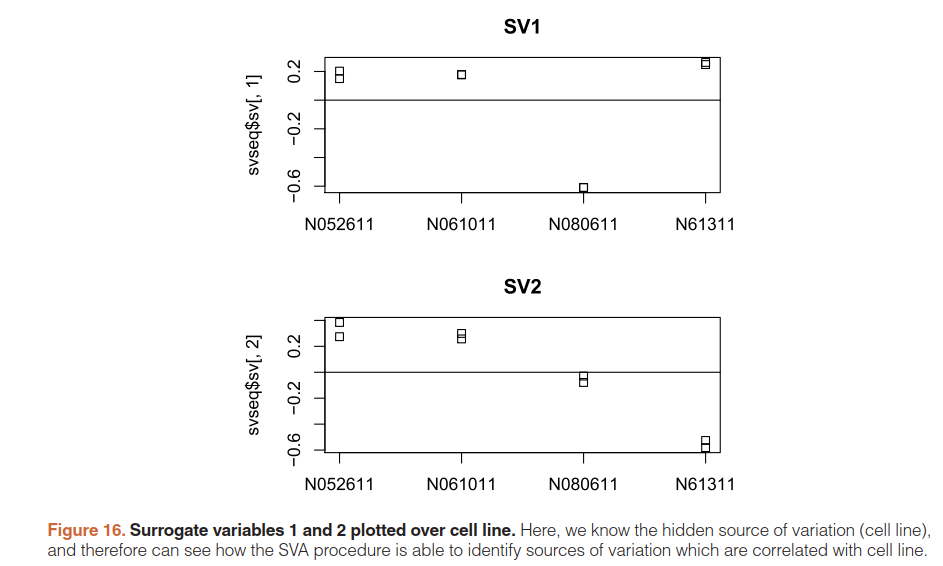
par(mfrow=c(2,1),mar=c(3,5,3,1))
stripchart(svseq$sv[,1] ~ dds$cell,vertical=TRUE,main="SV1")
abline(h=0)
stripchart(svseq$sv[,2] ~ dds$cell,vertical=TRUE,main="SV2")
abline(h=0)
ddssva <- dds
ddssva$SV1 <- svseq$sv[,1]
ddssva$SV2 <- svseq$sv[,2]
design(ddssva) <- ~ SV1 + SV2 + dex
ddssva <- DESeq(ddssva)
5.11)Time course experiments
详细看文档
library("fission")
data("fission")
ddsTC <- DESeqDataSet(fission, ~ strain + minute + strain:minute)
ddsTC <- DESeq(ddsTC, test="LRT", reduced = ~ strain + minute)
resTC <- results(ddsTC)
resTC$symbol <- mcols(ddsTC)$symbol
head(resTC[order(resTC$padj),],4)
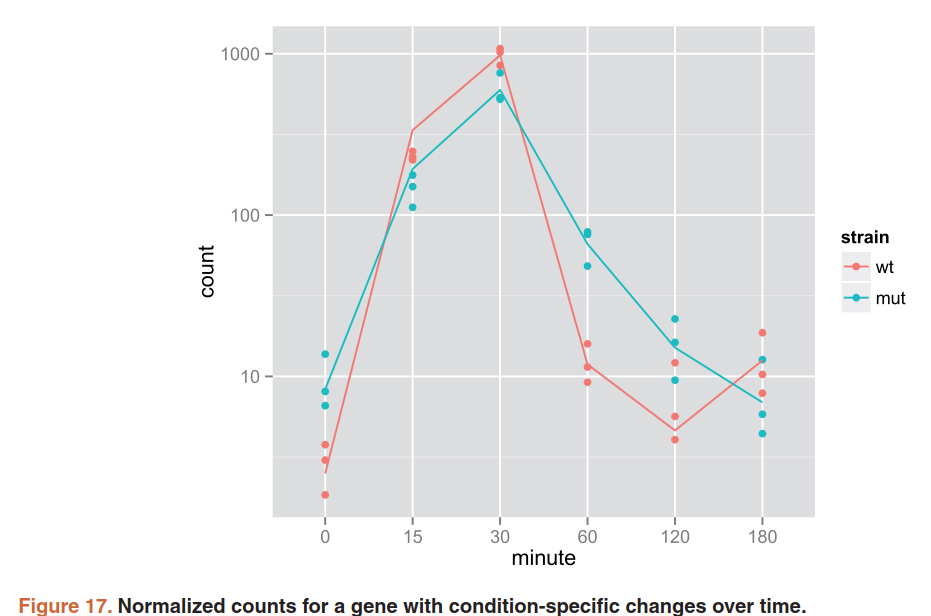
data <- plotCounts(ddsTC, which.min(resTC$padj),
intgroup=c("minute","strain"), returnData=TRUE)
ggplot(data, aes(x=minute, y=count, color=strain, group=strain)) +
geom_point() + stat_smooth(se=FALSE,method="loess") + scale_y_log10()
resultsNames(ddsTC)
res30 <- results(ddsTC, name="strainmut.minute30", test="Wald")
res30[which.min(resTC$padj),]
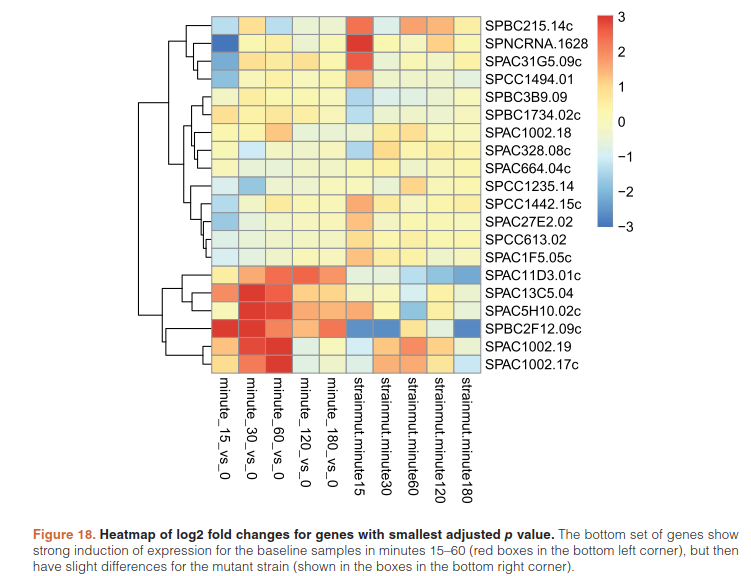
furthermore cluster significant genes by their profiles:
betas <- coef(ddsTC)
colnames(betas)
library("pheatmap")
topGenes <- head(order(resTC$padj),20)
mat <- betas[topGenes, -c(1,2)]
thr <- 3
mat[mat < -thr] <- -thr
mat[mat > thr] <- thr
pheatmap(mat, breaks=seq(from=-thr, to=thr, length=101),
cluster_col=FALSE)
airway之workflow的更多相关文章
- Mac 词典工具推荐:Youdao Alfred Workflow(可同步单词本)
想必大家都有用过 Mac 下常见的几款词典工具: 特性 系统 Dictionary 欧路词典 Mac 版 有道词典 Mac 版 在线搜索 ✗ ✔ ✔ 屏幕取词 ☆☆☆ ★★☆ ★☆☆ 划词搜索 ★★★ ...
- SharePoint 2013 create workflow by SharePoint Designer 2013
这篇文章主要基于上一篇http://www.cnblogs.com/qindy/p/6242714.html的基础上,create a sample workflow by SharePoint De ...
- Install and Configure SharePoint 2013 Workflow
这篇文章主要briefly introduce the Install and configure SharePoint 2013 Workflow. Microsoft 推出了新的Workflow ...
- Workflow笔记3——BookMark和持久化
BookMark 我们在平时的工作流使用中,并不是直接这样一气呵成将整个工作流直接走完的,通常一个流程到了某一个节点,该流程节点的操作人,可能并不会马上去处理该流程,而只有当处理人处理了该流程,流程才 ...
- Workflow笔记2——状态机工作流
状态机工作流 在上一节Workflow笔记1——工作流介绍中,介绍的是流程图工作流,后来微软又推出了状态机工作流,它比流程图功能更加强大. 状态机工作流:就是将工作流系统中的所有的工作节点都可以看做成 ...
- Workflow笔记1——工作流介绍
什么是工作流? 工作流(Workflow),是对工作流程及其各操作步骤之间业务规则的抽象.概括.描述.BPM:是Business Process Management的英文字母缩写.即业务流程管理,是 ...
- Rehosting the Workflow Designer
官方文档:https://msdn.microsoft.com/en-us/library/dd489451(v=vs.110).aspx The Windows Workflow Designer ...
- 工作流引擎Oozie(一):workflow
1. Oozie简介 Yahoo开发工作流引擎Oozie(驭象者),用于管理Hadoop任务(支持MapReduce.Spark.Pig.Hive),把这些任务以DAG(有向无环图)方式串接起来.Oo ...
- Creating a SharePoint Sequential Workflow
https://msdn.microsoft.com/en-us/library/office/hh824675(v=office.14).aspx Creating a SharePoint Seq ...
随机推荐
- 转载-JavaWeb学习总结
JavaWeb学习总结(五十三)——Web应用中使用JavaMail发送邮件 孤傲苍狼 2015-01-12 23:51 阅读:13407 评论:20 JavaWeb学习总结(五十二)——使用 ...
- Unreal Engine 4(虚幻UE4)GameplayAbilities 插件入门教程(六)GameplayEffect的级别设置
本节的内容不难,权当是复习.如果没有完成前面的教程,请前往学习. 第一步:用一个csv文件表示级别数据,下图中的Hurt随级别1~7表示其损伤值在1~7级别时分别是-7,-14,-20等.写好之后关闭 ...
- vmware12共享windows的文件给虚拟的linux
1:首先我的vmware的版本是12的 点击vmware的虚拟机---------------------->设置------------------------>选项---------- ...
- Web 项目遇到的乱码问题
问题代码: jsp: <%@ page language="java" contentType="text/html; charset=UTF-8" pa ...
- 小朋友学Python(1):Python简介与编程环境搭建
一.Python简介 不死Java,不朽C/C++,新贵Python. Python(英国发音:/ˈpaɪθən/ 美国发音:/ˈpaɪθɑːn/), 是一种面向对象的解释型计算机程序设计语言,由荷兰 ...
- IP分组交付和转发
1:交付 网络层监视底层物理网络对分组的处理过程叫做交付,分为直接交付和间接交付 1.1:直接交付 直接交付时,分组的终点是一台与交付着连接在同一个网络上的主机,发生在俩种情况下,分组的源点和终点都在 ...
- svn使用---在CentOS 7上搭建SVN服务器 及windows搭建svn步骤
svn搭建方法: https://blog.csdn.net/helijie92902/article/details/51935122?foxhandler=RssReadRenderProcess ...
- centos7配置vsftpd服务器
参考网站:https://blog.csdn.net/lianghongge/article/details/78209445 ==================================== ...
- JAVA 读取配置文件 xxx.properties
package config_demo; import java.io.InputStream; import java.util.Properties; public class UrlDemo { ...
- 使用Apache POI处理excel公式不更新的解决办法
使用poi更新excel时,如果单元格A设置了公式,当其依赖的其他单元格填充了值之后,导出的excel中A仍为公式而不是自动计算的值,如图: Paste_Image.png 分值小计没有更新成计算结果 ...
