DEXSeq
1)Introduction
DEXSeq是一种在多个比较RNA-seq实验中,检验差异外显子使用情况的方法。 通过差异外显子使用(DEU),我们指的是由实验条件引起的外显子相对使用的变化。 外显子的相对使用定义为:
number of transcripts from the gene that contain this exon / number of all transcripts from the gene
大致思想:. For each exon (or part of an exon) and each sample, we count how many reads map to this exon and how many reads map to any of the other exons of the same gene. We consider the ratio of these two counts, and how it changes across conditions, to infer changes in the relative exon usage
2)安装
if("DEXSeq" %in% rownames(installed.packages()) == FALSE) {source("http://bioconductor.org/biocLite.R");biocLite("DEXSeq")}
suppressMessages(library(DEXSeq))
ls('package:DEXSeq')
pythonScriptsDir = system.file( "python_scripts", package="DEXSeq" )
list.files(pythonScriptsDir)
## [1] "dexseq_count.py" "dexseq_prepare_annotation.py" #查看是否含有这两个脚本
python dexseq_prepare_annotation.py Drosophila_melanogaster.BDGP5.72.gtf Dmel.BDGP5.25.62.DEXSeq.chr.gff #GTF转化为GFF with collapsed exon counting bins.
python dexseq_count.py Dmel.BDGP5.25.62.DEXSeq.chr.gff untreated1.sam untreated1fb.txt #count
3) 用自带实验数据集(数据预处理)
suppressMessages(library(pasilla))
inDir = system.file("extdata", package="pasilla")
countFiles = list.files(inDir, pattern="fb.txt$", full.names=TRUE) #countfile(如果不是自带数据集,可以由dexseq_count.py脚本生成)
basename(countFiles)
flattenedFile = list.files(inDir, pattern="gff$", full.names=TRUE)
basename(flattenedFile) #gff文件(如果不是自带数据集,可以由dexseq_prepare_annotation.py脚本生成)
########构造数据框sampleTable,包含sample名字,实验,文库类型等信息#######################
sampleTable = data.frame(
row.names = c( "treated1", "treated2", "treated3",
"untreated1", "untreated2", "untreated3", "untreated4" ),
condition = c("knockdown", "knockdown", "knockdown",
"control", "control", "control", "control" ),
libType = c( "single-end", "paired-end", "paired-end",
"single-end", "single-end", "paired-end", "paired-end" ) )
sampleTable ##############构建 DEXSeqDataSet object#############################
dxd = DEXSeqDataSetFromHTSeq(
countFiles,
sampleData=sampleTable,
design= ~ sample + exon + condition:exon,
flattenedfile=flattenedFile ) #四个参数
4)Standard analysis work-flow
########以下是简单的实验设计#####
genesForSubset = read.table(file.path(inDir, "geneIDsinsubset.txt"),stringsAsFactors=FALSE)[[1]] #基因子集ID
dxd = dxd[geneIDs( dxd ) %in% genesForSubset,] #取子集,减少运行量
head(colData(dxd))
head( counts(dxd), 5 )
split( seq_len(ncol(dxd)), colData(dxd)$exon )
sampleAnnotation( dxd )
############# dispersion estimates and the size factors#############
dxd = estimateSizeFactors( dxd ) ##Normalisation
dxd = estimateDispersions( dxd )
plotDispEsts( dxd ) #图1 #################Testing for differential exon usage############
dxd = testForDEU( dxd )
dxd = estimateExonFoldChanges( dxd, fitExpToVar="condition")
dxr1 = DEXSeqResults( dxd )
dxr1
mcols(dxr1)$description
table ( dxr1$padj < 0.1 )
table ( tapply( dxr1$padj < 0.1, dxr1$groupID, any ) )
plotMA( dxr1, cex=0.8 ) #图2

To see how the power to detect differential exon usage depends on the number of reads that map to an exon, a so-called MA plot is useful, which plots the logarithm of fold change versus average normalized count per exon and marks by red colour the exons which are considered significant; here, the exons with an adjusted p values of less than 0.1

############以下是更复杂的实验设计##################
formulaFullModel = ~ sample + exon + libType:exon + condition:exon
formulaReducedModel = ~ sample + exon + libType:exon
dxd = estimateDispersions( dxd, formula = formulaFullModel )
dxd = testForDEU( dxd,
reducedModel = formulaReducedModel,
fullModel = formulaFullModel )
dxr2 = DEXSeqResults( dxd )
table( dxr2$padj < 0.1 )
table( before = dxr1$padj < 0.1, now = dxr2$padj < 0.1 )##和简单的实验设计比较
5)Visualization
plotDEXSeq( dxr2, "FBgn0010909", legend=TRUE, cex.axis=1.2, cex=1.3,
lwd=2 )
plotDEXSeq( dxr2, "FBgn0010909", displayTranscripts=TRUE, legend=TRUE,
cex.axis=1.2, cex=1.3, lwd=2 )
plotDEXSeq( dxr2, "FBgn0010909", expression=FALSE, norCounts=TRUE,
legend=TRUE, cex.axis=1.2, cex=1.3, lwd=2 )
plotDEXSeq( dxr2, "FBgn0010909", expression=FALSE, splicing=TRUE,
legend=TRUE, cex.axis=1.2, cex=1.3, lwd=2 )
DEXSeqHTML( dxr2, FDR=0.1, color=c("#FF000080", "#0000FF80") )


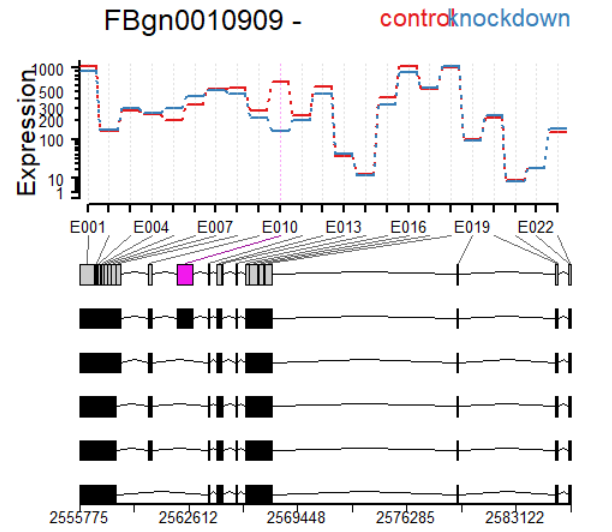
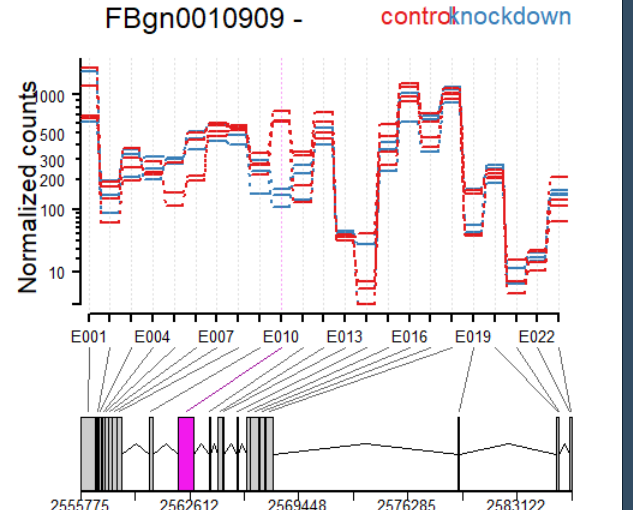
DEXSeq的更多相关文章
- 【转录组入门】6:reads计数
作业要求: 实现这个功能的软件也很多,还是烦请大家先自己搜索几个教程,入门请统一用htseq-count,对每个样本都会输出一个表达量文件. 需要用脚本合并所有的样本为表达矩阵.参考:生信编程直播第四 ...
- Bulk RNA-Seq转录组学习
与之对应的是single cell RNA-Seq,后面也会有类似文章. 参考:https://github.com/xuzhougeng/Learn-Bioinformatics/ 作业:RNA-s ...
- Bioconductor应用领域之基因芯片
引用自https://mp.weixin.qq.com/s?__biz=MzU4NjU4ODQ2MQ==&mid=2247484662&idx=1&sn=194668553f9 ...
随机推荐
- Juery 实现淡出 淡现效果
HTML: <!DOCTYPE html PUBLIC "-//W3C//DTD XHTML 1.0 Transitional//EN" "http://www.w ...
- 织梦调用文章 ID (来源:百度知道)
问:{dede:field.id /} {dede:channel type='son' orderby='sortrank'} <a href='[field:typeurl/]'>&l ...
- 跟我一起学Makefile
概述 什么是makefile?或许很多Winodws程序员都不知道这个东西,因为那些Windows IDE都为你做了这个工作,但我觉得要做一个好的和professional的程序员,makefile还 ...
- BASIC-23_蓝桥杯_芯片测试
思路: 1.当测试与被测试的芯片全部可以互相测试时,为好芯片; 示例代码: #include <stdio.h>#define N 20 int main(void){ int n = 0 ...
- bzoj4161: Shlw loves matrixI
Description 给定数列 {hn}前k项,其后每一项满足 hn = a1*h(n-1) + a2*h(n-2) + ... + ak*h(n-k) 其中 a1,a2...ak 为给定数列.请计 ...
- 9.MVC模式 -- 改造源代码
一.MVC设计模式 软件可以认为有 Model View Controller 来组成 MVC设计模式 要求这三部分 应该尽量独立 互不干扰 使程序结构清晰 便于开发和维护 二.JAVAEE经典三层 ...
- 开发框架-移动开发平台: mPaaS
ylbtech-开发框架-移动开发平台: mPaaS 移动开发平台 mPaaSmPaaS(Mobile PaaS)为 App 开发.测试.运营及运维提供云到端的一站式解决方案,能有效降低技术门槛.减少 ...
- JAVA的非对称加密算法RSA——加密和解密
原文转载至:https://www.cnblogs.com/OnlyCT/p/6586856.html 第一部分:RSA算法原理与加密解密 一.RSA加密过程简述 A和B进行加密通信时,B首先要生成一 ...
- php array_flip() 删除数组重复元素——大彻大悟
1. php array_flip() 删除数组重复元素,如果用于一维索引数组,好理解. [root@BG-DB:~]$more arr.php <?php $arr = ar ...
- (转)手机的AP和BP是什么?
AP:Application Processor,即应用芯片 BP:Baseband Processor,即基带芯片 搞什么嘛,双核就双核呗,怎么又搞出个AP和BP啊 原来,FCC(美国联邦通信委员会 ...
