edgeR
1)简介
edgeR作用对象是count文件,rows 代表基因,行代表文库,count代表的是比对到每个基因的reads数目。它主要关注的是差异表达分析,而不是定量基因表达水平。
edgeR works on a table of integer read counts, with rows corresponding to genes and columns to independent libraries. The counts represent the total number of reads aligning to each gene (or other genomic locus).edgeR is concerned with differential expression analysis rather than with the quantification of expression levels. It is concerned with relative changes in expression levels between conditions,but not directly with estimating absolute expression levels.
edgeR作用的是真实的比对统计,因此不建议用预测的转录本
Note that edgeR is designed to work with actual read counts. We not recommend that predicted transcript abundances are input the edgeR in place of actual counts.
归一化原因:
技术原因影响差异表达分析:
1)Sequencing depth:统计测序深度(即代表的是library size);
2)RNA composition:个别异常高表达基因导致其它基因采样不足
3)GC content: sample-specific effects for GC-content can be detected
4)sample-specific effects for gene length have been detected
注意:edgeR必须是原始表达量,而不能是rpkm等矫正过的。
Note that normalization in edgeR is model-based, and the original read counts are not themselves transformed. This means that users should not transform the read counts in any way before inputing them to edgeR. For example, users should not enter RPKM or FPKM values to edgeR in place of read counts. Such quantities will prevent edgeR from correctly estimating the mean-variance relationship in the data, which is a crucial to the statistical strategies underlying edgeR.Similarly, users should not add artificial values to the counts before inputing them to edgeR.
2)安装
if("edgeR" %in% rownames(installed.packages()) == FALSE) {source("http://bioconductor.org/biocLite.R");biocLite("edgeR")}
suppressMessages(library(edgeR))
ls('package:edgeR')
3)矩阵构建及差异分析
需要构建2个矩阵:1、表达矩阵;2、分组矩阵( 实验设计);
-------------------------------------------------------表达矩阵-----------------------------------------
3.1、读取表达矩阵文件(Reading in the data)
#读取文件
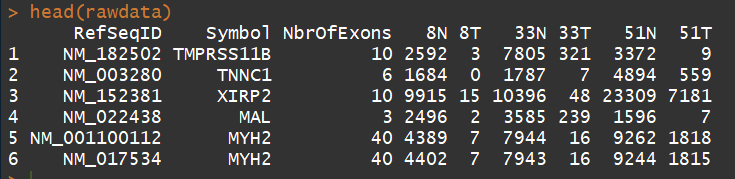
rawdata <- read.delim("E:/software/R/R-3.5.0/library/edgeR/Meta/TableS1.txt", check.names=FALSE, stringsAsFactors=FALSE)
head(rawdata)
3.2 、构建DGEList对象
这里因为已经有rawdata的count文件,因此直接用DGEList()函数就行了,否则要用readDGE()函数
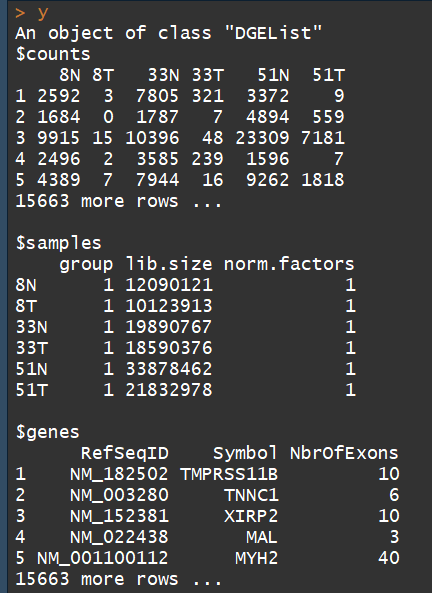
y <- DGEList(counts=rawdata[,4:9], genes=rawdata[,1:3])##构建DGEList对象
DGEList对象主要有三部分:
1、counts矩阵:包含的是整数counts;
2、samples数据框:包含的是文库(sample)信息。包含 lib.size列 :for the library size (sequencing depth) for each sample,如果不自定义, the library sizes will be computed from the column sums of the counts。其中还有一个group列,用于指定每个sample组信息
3、一个可选的数据框genes:gene的注释信息
3.3)数据注释( Annotation)
这里主要是因为该文章数据是前好多年的,因此需要过滤,symbol更新等。
1)The study was undertaken a few years ago, so not all of the RefSeq IDs provided by match RefSeq IDs currently in use. We retain only those transcripts with IDs in the current NCBI annotation, which is provided by the org.HS.eg.db package
2)因为edgeR默认使用NCBI中refSeq的ID,所以通过refseq Id 找到entrezID,然后通过entrezID对symbol更新
#######retain only those transcripts with IDs in the current NCBI annotation provided by the org.HS.eg.db######
library(org.Hs.eg.db)
idfound <- y$genes$RefSeqID %in% mappedRkeys(org.Hs.egREFSEQ)
y <- y[idfound,]
dim(y) ##15550 6
###################### 在注释中加入 Entrez Gene IDs #########################
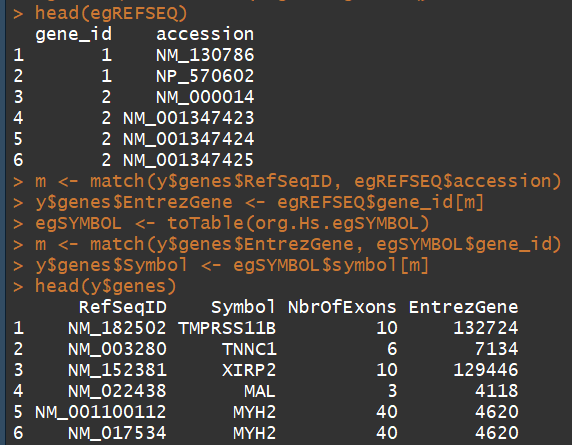
egREFSEQ <- toTable(org.Hs.egREFSEQ)
m <- match(y$genes$RefSeqID, egREFSEQ$accession)
y$genes$EntrezGene <- egREFSEQ$gene_id[m]
#####################用Entrez Gene IDs更新gene symbols##########################
egSYMBOL <- toTable(org.Hs.egSYMBOL)
m <- match(y$genes$EntrezGene, egSYMBOL$gene_id)
y$genes$Symbol <- egSYMBOL$symbol[m]
head(y$genes)
3.4) 过滤和归一化(Filtering and normalization)
过滤一:Different RefSeq transcripts for the same gene symbol count predominantly the same reads. So we keep one transcript for each gene symbol. We choose the transcript with highest overall count:
o <- order(rowSums(y$counts), decreasing=TRUE)
y <- y[o,]
d <- duplicated(y$genes$Symbol)
y <- y[!d,]
nrow(y)
过滤二:Normally we would also filter lowly expressed genes.For this data, all transcripts already have at least 50 reads for all samples of at least one of the tissues types.
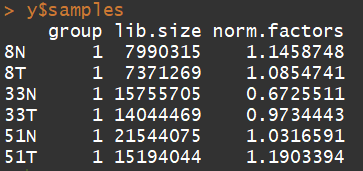
y$samples$lib.size <- colSums(y$counts) #Recompute the library sizes
###############################Use Entrez Gene IDs as row names:#####################
rownames(y$counts) <- rownames(y$genes) <- y$genes$EntrezGene
y$genes$EntrezGene <- NULL
归一化:TMM normalization is applied to this dataset to account for compositional difference between the libraries.
y <- calcNormFactors(y)
y$samples
3.5) 数据的探索(Data exploration)
样本间关系(samples for outliers and for other relationships)
plotMDS(y)
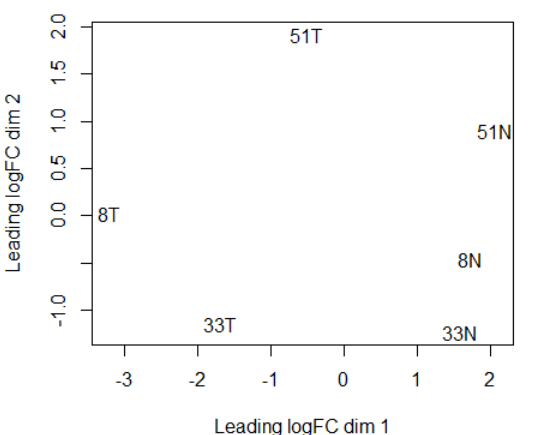
PC1将tumor和nomal组分开,PC2 大略和病号对应。也侧面体现了肿瘤组的异质性
--------------------------分组矩阵(根据实验设计、目的)--------------------------------
Here we want to test for differential expression between tumour and normal tissues within patients, i.e. adjusting for differences between patients.
Patient <- factor(c(8,8,33,33,51,51))
Tissue <- factor(c("N","T","N","T","N","T"))
data.frame(Sample=colnames(y),Patient,Tissue)
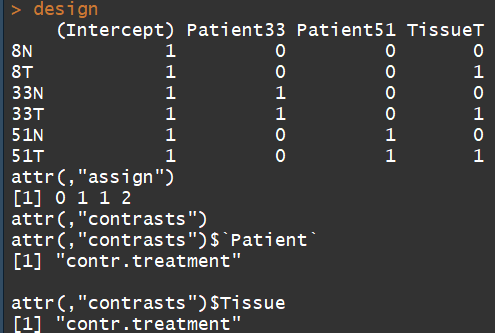
design <- model.matrix(~Patient+Tissue)
rownames(design) <- colnames(y)
design
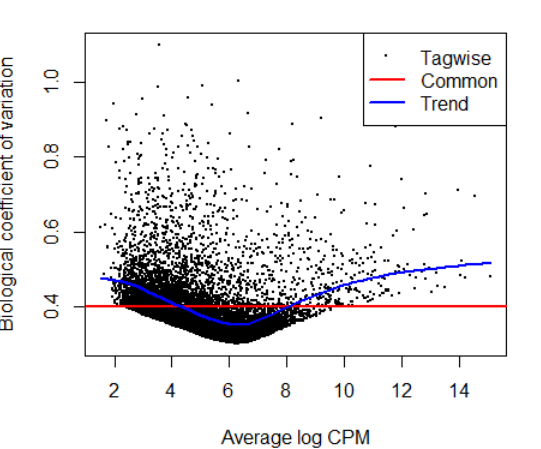
3.4)Estimating the dispersion(estimate the NB dispersion for the dataset.)
y <- estimateDisp(y, design, robust=TRUE)
y$common.dispersion #0.1594505
plotBCV(y)
-----------------------------------差异分析-----------------------------------------
3.5) 差异分析(Differential expression)
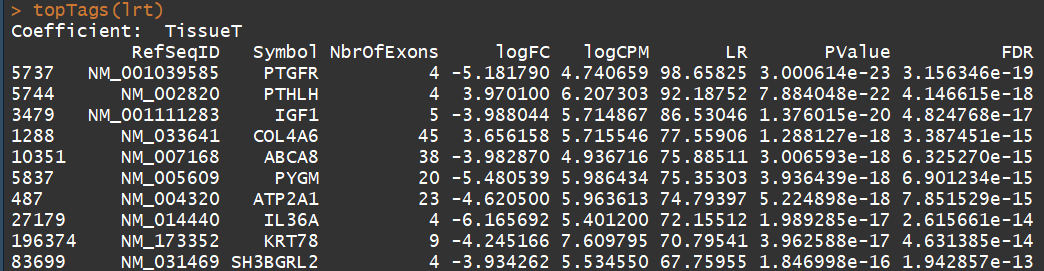
fit <- glmFit(y, design)
lrt <- glmLRT(fit)
topTags(lrt)
summary(decideTests(lrt))
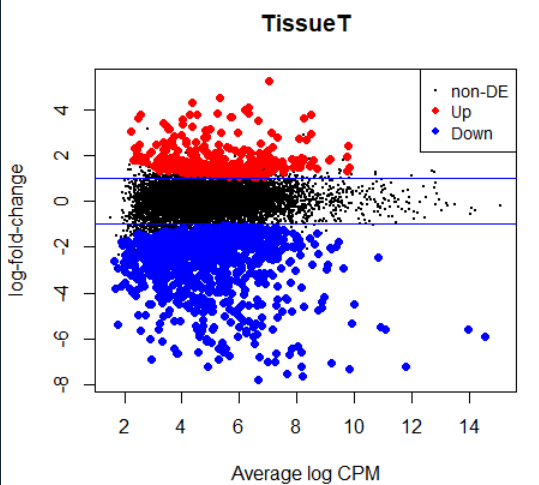
plotMD(lrt)
abline(h=c(-1, 1), col="blue")

------------------------------- Gene ontology analysis----------------------------------------
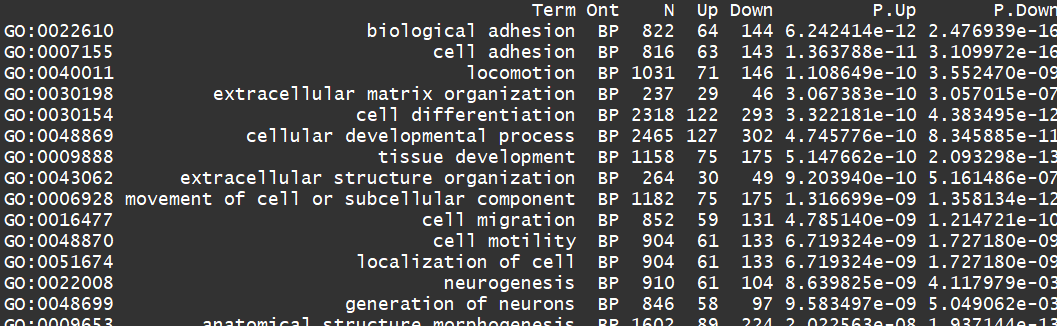
对上调的基因进行BP分析
go <- goana(lrt)
topGO(go, ont="BP", sort="Up", n=30)
edgeR的更多相关文章
- cuffdiff 和 edgeR 对差异表达基因的描述
ASE又走到了关键的一步 要生成能决定是否有差异表达的table. 准备借鉴一下cuffdiff和edgeR 的结果 cuffdiff对差异表达基因的描述: 一共十四列: 第一列, test_id ...
- 简单使用DESeq2/EdgeR做差异分析
简单使用DESeq2/EdgeR做差异分析 Posted: 五月 07, 2017 Under: Transcriptomics By Kai no Comments DESeq2和EdgeR都 ...
- 使用limma、Glimma和edgeR,RNA-seq数据分析易如反掌
使用limma.Glimma和edgeR,RNA-seq数据分析易如反掌 Charity Law1, Monther Alhamdoosh2, Shian Su3, Xueyi Dong3, Luyi ...
- edgeR使用学习【转载】
转自:http://yangl.net/2016/09/27/edger_usage/ 1.Quick start 2. 利用edgeR分析RNA-seq鉴别差异表达基因: #加载软件包 librar ...
- 用TCGA收集的mRNA表达数据作差异表达
做差异表达的软件DEseq和edgeR所需要的数据格式必须是原始counts,经过normalization和log2后的数据都不适合,所以对于做差异表达计算的童鞋可以使用ExperimentHub下 ...
- sql是最成功的第四代语言
SQL发展的前世今生 很多年前,两名年轻的IBM研究员将一门关系型语言带到了数据库领域,旨在使用声明性的方式来操作数据.从Don Chamberlin和Ramond Boyce发表"SEQU ...
- RNA-seq标准化
你的 heatmap 可能用错数据了 (组间表达量标准化) http://www.genek.tv/article/24 RNA-seq的标准化方法罗列 https://www.jianshu.com ...
- 史上最全 | 39个RNAseq分析工具与对比
文献:Sahraeian S M E, Mohiyuddin M, Sebra R, et al. Gaining comprehensive biological insight into the ...
- RNA-seq中的基因表达量计算和表达差异分析
RNA-seq中的基因表达量计算和表达差异分析 差异分析的步骤:1)比对:2) read count计算:3) read count的归一化:4)差异表达分析: 背景知识:1)比对:普通比对: BWA ...
随机推荐
- Centos替换默认源
将默认的国外源替换为国内的网易的源 参考帮助文档:http://mirrors.163.com/.help/centos.html 查看本机版本:cat /etc/redhat-release 先安装 ...
- 【Spring-AOP-学习笔记-5】@AfterReturning增强处理简单示例
项目结构 业务代码 @Component("hello") public class HelloImpl implements Hello { // 定义一个简单方法,模拟 ...
- 【Hibernate学习笔记-5】@Formula注解的使用
ORM映射关系:注解方式 package org.crazyit.app.domain; import javax.persistence.*; import org.hibernate.annota ...
- Spring中的后置处理器BeanPostProcessor讲解
Spring中提供了很多PostProcessor供开发者进行拓展,例如:BeanPostProcessor.BeanFactoryPostProcessor.BeanValidationPostPr ...
- js中一些对字符串的操作等
看代码时候,发现一些写的很好的js对字符串的操作,记录下来,持续更新等>... js trim()的实现: function trim(string){ return string.replac ...
- 转转转!java继承中的this和super
学习java时看了不少尚学堂马士兵的视频,还是挺喜欢马士兵的讲课步骤的,二话不说,先做实例,看到的结果才是最实际的,理论神马的全是浮云.只有在实际操作过程中体会理论,在实际操作过程中升华理论才是最关键 ...
- [html] 回到页首
[转]本文来自:最简单最强大的插件框架(Net 2.0+) http://www.cnblogs.com/baihmpgy/p/3305215.html <!doctype html> & ...
- [转]将字体嵌入程序资源中 C# Winform
http://social.msdn.microsoft.com/Forums/officeapps/zh-CN/61b717ae-f925-443a-baad-2b85f2564826/cwinfo ...
- 用linux的iconv函数 转换编码
inux shell 配置文件中默认的字符集编码为UTF-8 .UTF-8是unicode的一种表达方式,gb2312是和unicode都是字符的编码方式,所以说gb2312跟utf-8的概念应该不是 ...
- 阿里云上部署tomcat启动后,通过http不能访问
原因是因为阿里为了安全设置了安全组策略,必须我们授权的端口,其他计算机才能通过http访问 设置流程: 点击安全组 再点击:配置规则 然后点击:添加安全组规则 开始配置:划红线的必写,授权对象:0.0 ...
